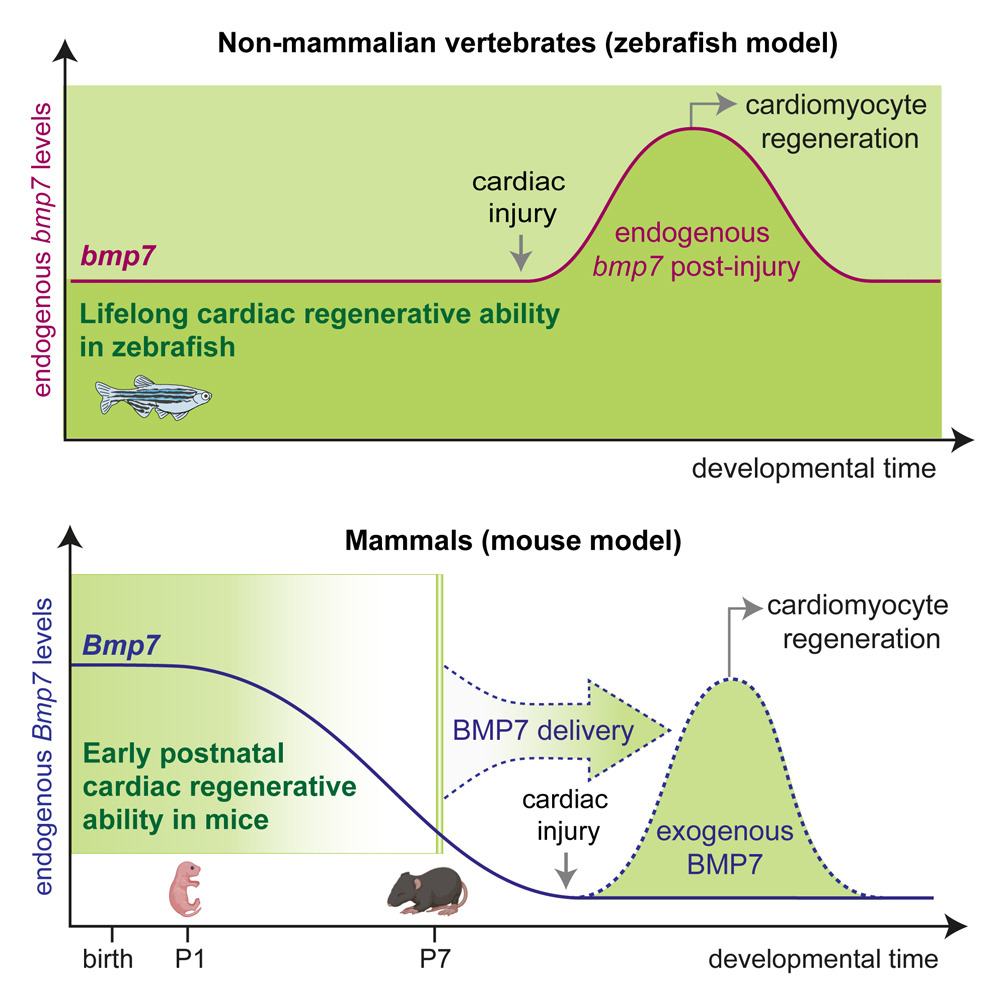
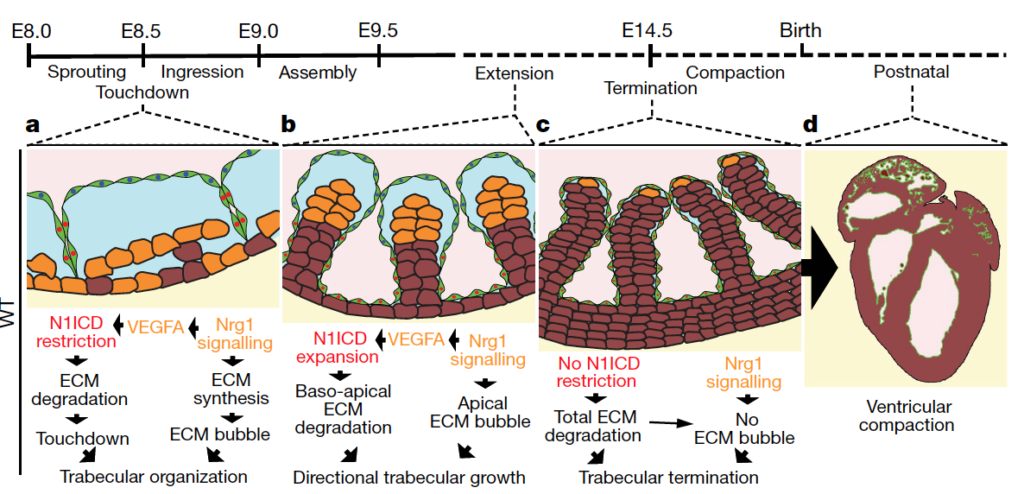
We are thrilled to announce our latest publication in Cell Reports! Our research indicates that a decrease in the production of growth factors, particularly BMP7, during early postnatal development contributes to the loss of regenerative capacity of the mammalian heart.
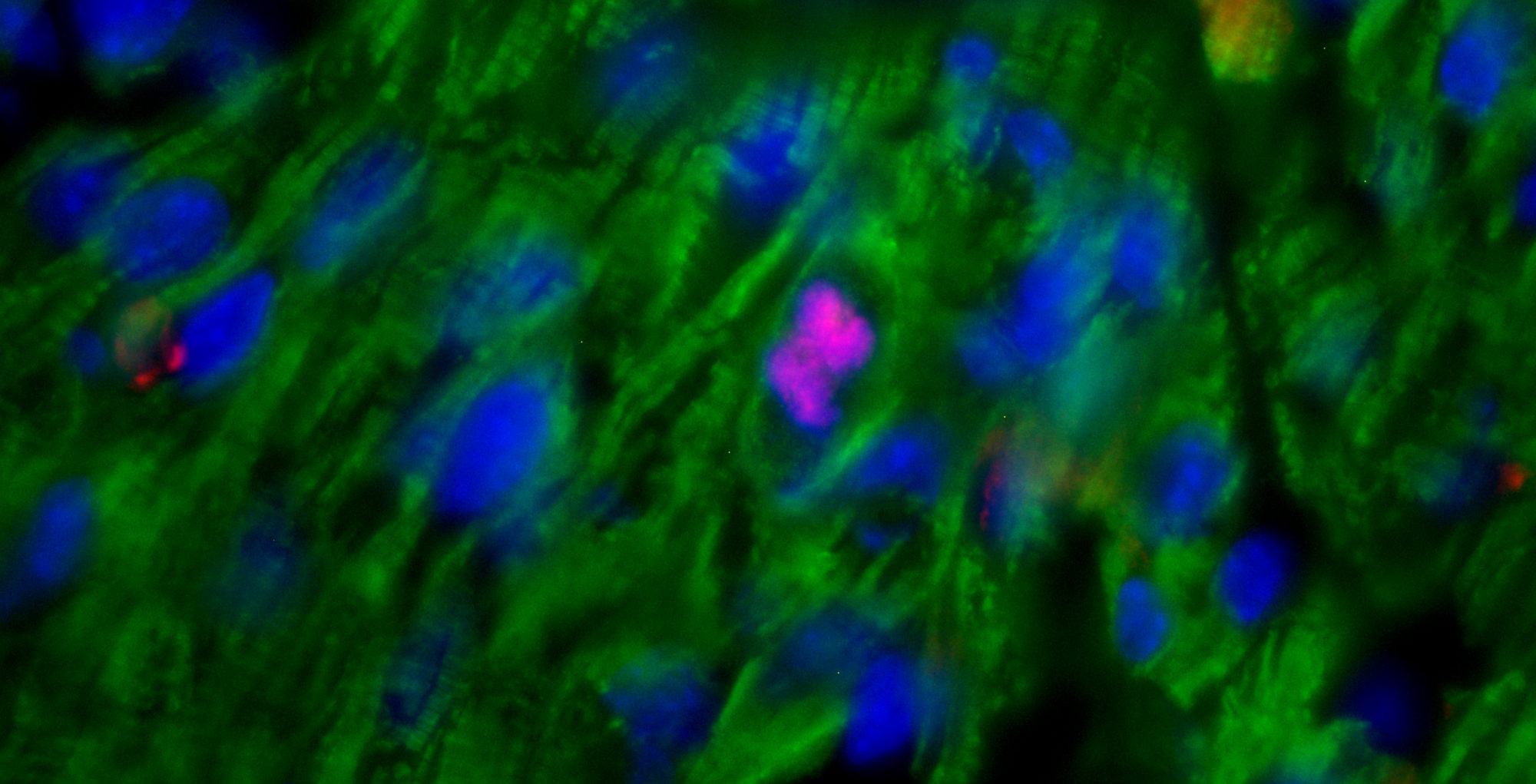
We demonstrate that BMP7 supports cardiomyocyte proliferation during the neonatal stage, and its administration boosts cardiomyocyte proliferation in postnatal life, even in adulthood, and especially after myocardial infarction. These findings suggest that BMP7 administration holds promise as a therapeutic approach for heart regeneration. Moreover, our study finds support from the zebrafish model, which naturally regenerates injured hearts. In this regard, inhibiting BMP7 impeded cardiomyocyte regeneration post-cardiac injury, while its induction accelerated the process. We believe our findings pave the way for heart regenerative therapies based on the administration of BMP7.
Congratulations to Chiara Bongiovanni for leading the project, and congratulations to the other lab team members Irene Del Bono, Carmen Miano, Stefano Boriati, Silvia Da Pra, Francesca Sacchi, Francesca Pontis, and Ilaria Petraroia for their help and support in experimental activities. We express our gratitude to collaborators who were instrumental in the success of this project, particularly the research groups led by Eldad Tzahor(Weizmann Institute of Science, Israel), Gilbert Weidinger (University of Ulm, Germany), Stephan Heermann (University of Friburg, Germany), Mattia Lauriola, and Carlo Ventura (University of Bologna, Italy).
Original Article Link (open access): https://dx.doi.org/10.1016/j.celrep.2024.114162